TECHNOLOGY
AI Joins the Fight Against Obesity
FDA clears Dexcom and Signos tools as insurers and clinicians weigh digital alternatives to costly weight-loss drugs
21 Aug 2025
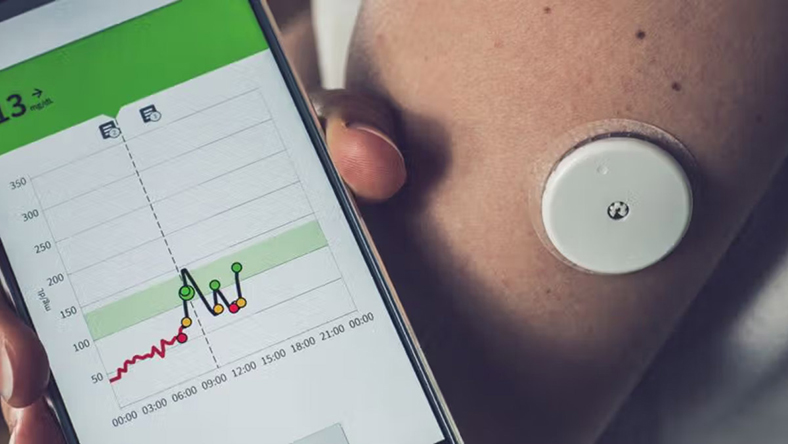
The US Food and Drug Administration has cleared two connected technologies that bring artificial intelligence into obesity treatment, marking a shift in how chronic weight management is approached.
Dexcom’s Stelo, the first over-the-counter continuous glucose monitor, received clearance in March 2024. A year later, regulators approved Signos’s AI-driven glucose system, announced publicly in August 2025. Used together, the devices provide a flow of real-time glucose data that Signos software translates into personalised guidance on food, exercise and lifestyle.
Rather than relying only on clinic visits, users receive instant digital prompts designed to help form healthier habits and reduce or delay reliance on GLP-1 weight-loss drugs. The company offers access through subscription plans, positioning the system as a consumer-facing digital coach.
The technology reflects a broader trend toward personalised and data-led health care. Employers and insurers seeking to manage rising obesity-related costs may view such systems as scalable complements to drug therapy. Clinicians could also draw on continuous patient data to refine treatment, while technology and pharmaceutical groups explore partnerships at the intersection of devices, AI and therapeutics.
Regulatory backing suggests growing confidence in technology's role in chronic disease management. Still, questions remain over insurance coverage, long-term clinical validation and patient data privacy.
If adoption expands, the Dexcom-Signos combination could mark an early stage in the integration of connected devices and AI into obesity treatment, signalling a move toward more sustainable models of care.
Latest News
25 Feb 2026
Can AI Rethink How New Drugs Are Found?24 Feb 2026
Lilly Deepens AI Push in Obesity Drug Race23 Feb 2026
Another Shot in the Obesity Gold Rush19 Feb 2026
Precision Medicine Rises as Biomarkers Drive Change
Related News

INNOVATION
25 Feb 2026
Can AI Rethink How New Drugs Are Found?

PARTNERSHIPS
24 Feb 2026
Lilly Deepens AI Push in Obesity Drug Race

INVESTMENT
23 Feb 2026
Another Shot in the Obesity Gold Rush
SUBSCRIBE FOR UPDATES
By submitting, you agree to receive email communications from the event organizers, including upcoming promotions and discounted tickets, news, and access to related events.